Zeolite
ZeolithMED detox powder 400g, medical device with CE certificate
100% natural clinoptiolite zeolite powder for 2 months
- save 1% buy 2 for €20.61 each
- save 4% buy 4 for €19.98 each
- save 8% buy 6 for €19.25 each
Ratings & Reviews
What is Zeolite?
Author: Zeolite bentonite shipping, updated: 09.12.2019
Detoxify with clinoptilolite zeolite in a natural way - ingestible zeolite
You are on the safe side here, because here you receive clinoptilolite zeolite for human consumption as quality tested and CE-certified medical devices directly from us, the manufacturer. If you have any questions, please contact us by e-mail. Information on our payment options can be found here. We endeavour to ship all orders received by 14.30 Mon - Fri on the same day.
The natural mineral zeolite is a volcanic rock with a high silicon content. There are more than 45 different naturally occurring zeolites, of which the clinoptilolite zeolite in particular is suitable for ingestion in the form of CE-certified medical devices such as our zeolite MED. To make it more effective, the clinoptilolite zeolite is micronized and activated. The indigestible zeolite MED migrates through the gastrointestinal tract, binds pollutants selectively like a sponge and is excreted naturally with the stool through the intestine without burdening the metabolism. The body is detoxified in a natural way.
Clinoptilolite zeolite: 100 percent natural
Clinoptilolite zeolite is available as ZeolithMED Detox powder, ultra-fine powder and in capsules as well as ZeolithMED skin cream, toothpaste and hyaluronic gel. For beginners and all who want to try out natural detoxification with clinoptilolite zeolite and bentonite for the first time, we offer the Info-Test-Set Powder and Info-Test-Set ultra-fine Powder. Our medical device ZeolithMED is a 100 percent pure natural mineral of volcanic origin, which is supplied nanoparticle-free, without further additives or admixtures.
Do you still have questions? Answers to frequently asked questions about detoxification with Zeolite, Bentonite and ZeoBent can be found in our FAQs.
Zeolite as powder, fine powder, capsules or skin care?
 PDF Product Informationen
PDF Product Informationen
 PDF Instructions for use Zeolite MED
PDF Instructions for use Zeolite MED
What is Zeolite?
Author: Zeolith Bentonit Versand, updated: 03.11.2023
Zeolite clinoptilolite actively supports detoxify the body from heavy metals
Zeolite is a naturally occurring volcanic rock and the only ingredient of ZeoliteMED. In the form of powder and capsules, it actively supports the detoxification of the body from harmful substances. Its use can reduce symptoms such as fatigue and headaches associated with elevated levels of heavy metals. ZeolithMED is an independently tested product for long-term, oral use in humans.
The action of zeolite is based on adsorption and ion exchange. The natural mineral zeolite is indigestible and can selectively bind toxins such as certain heavy metals in the gastrointestinal tract of humans on its porous, sponge-like surface. The toxins bound to the zeolite in the gastrointestinal tract are thus removed from the body via the intestine even before they are absorbed through the intestine. Toxins include heavy metals such as lead, mercury, chromium, cadmium and nickel, as well as aluminum. The efficacy and safety of zeolite under the brand name ZeolithMED has been tested and confirmed during medical device approval.
Clinoptilolite zeolite: 100 percent natural
Clinoptilolite zeolite is available as ZeoliteMED Detox Powder, ultrafine powder and in capsules as well as in products from the category Body Care as ZeoliteMED skin cream and toothpaste. For beginners and all those who would like to try natural detoxification with clinoptilolite-zeolite and bentonite for the first time, we offer the Test Set Detox POWDER and Test Set Detox POWDER ultrafine. Our ZeoliteMED is a 100 percent pure natural mineral of volcanic origin that is supplied nanoparticle-free, without any other additives or admixtures.
Do you have any questions? Answers to frequently asked questions about detoxification with zeolite, bentonite and ZeoBent can be found in our FAQs.
Buy zeolite as a powder, ultra-fine powder, capsule or natural cosmetics?
 PDF Product Informationen
PDF Product Informationen
 PDF Instructions for use Zeolite MED
PDF Instructions for use Zeolite MED
- Zeolite
- Binding pollutants with zeolite
- Zeolite: natural stone with high binding power
- Also a kind of healing clay: zeolite
- What is adsorption and what does zeolite have to do with it?
- How does adsorption with zeolite work for humans?
- Effect of Zeolite
- Zeolite as a prophylactic
- Zeolite effect – alleviation of symptoms
- Zeolite as an antioxidant
- Which illnesses can Zeolite help?
- Why do we need help in coping with pollutants?
- Relieving the liver - keep toxins away
- Dangers from heavy metals in everyday life
- Where do we come across lead?
- What are the symptoms and dangers of lead poisoning?
- What are the symptoms and dangers of mercury poisoning?
- What are the symptoms and dangers of cadmium poisoning?
- What are the symptoms and dangers of ammonium poisoning?
- Zeolite Powder, Ultra Fine Powder or Capsules?
- Effect of Zeolite natural cosmetics (external use)
- Zeolite interactions with other medicines
- Side effects of Zeolite
- Warnings and precautions
- Use and dosage of Zeolite
Zeolite
ZeolithMED binds unavoidable pollutants in a natural way and thus makes an important contribution to your health. Symptoms whose causes can be traced back to exposure to harmful substances can be alleviated and reduced with ZeoliteMED. ZeolithMED, a natural product made from the natural mineral zeolite, can bind and drain harmful substances in the gastrointestinal tract like a sponge. The indigestible zeolite MED, which is loaded with harmful substances, is naturally excreted in the stool via the intestines without burdening the metabolism.
Binding pollutants with zeolite
Natural zeolite powder acts like a sponge and can help relieve the body.
Zeolite: natural stone with high a binding power
Since the Middle Ages, healing clay has been used as a natural remedy for a variety of health complaints. Depending on the area of application and variant, healing clay is composed of powdered rock with various components, such as loess, peat, loam, clay, moor and others.
Also a kind of healing clay: Zeolite
Natural zeolite can also be used to support health. Zeolite is a natural rock of volcanic origin. The surface of the silicon-rich mineral is particularly capable of binding pollutants. The process that takes place is called adsorption.
What is adsorption and what does zeolite have to do with it?
Adsorption is a physical process in which substances stick to the surface of another substance and accumulate on it.
Due to its microporous surface and its ability to exchange ions, zeolite has a lot of potential for the adhesion of other substances. As a result, it can act like a sponge and, in particular, bind pollutants.
In use for a long time
Already in ancient times, natural zeolite was used for detoxification in the form of clinoptilolite. After the Chernobyl reactor disaster, the building was encased in a cement belt, which was mixed with tons of zeolite. The powdered mineral was also used to decontaminate people and the environment.
How does zeolite adsorption work for humans?
When zeolite is used medicinally, it is used as clinoptilolite - a natural, microporous tuff rock. The natural zeolite has a crystal lattice structure with many cavities. The latter contain cations such as calcium, sodium, magnesium and potassium.
Due to its structure, zeolite can adsorb substances on the one hand and achieve ion exchange on the other: Adsorption causes foreign substances to adhere to the surface of the rock. Something similar can also take place in the course of ion exchange: In this process, the cations of the mineral can be released and replaced by pollutant ions in the body.
Like a sponge, the mineral is able to bind harmful substances in the digestive tract and remove them from the organism in a natural way. Due to this special nature, zeolite can make an important contribution to health. Zeolite itself is indigestible and is excreted through the intestines.
The effect of Zeolite
Zeolite works through the binding (adsorption) of:
- Mercury
- Lead
- Cadmium
- Aluminium
The CE-approved medical device ZeolithMED can be used to relieve the organism by detoxifying the intestinal tract.
The effect of zeolite is based, among other things, on its special property of binding harmful substances such as aluminium (Al3+), lead (Pb2+) and mercury (Hg2+) through selective ion exchange and adsorption.
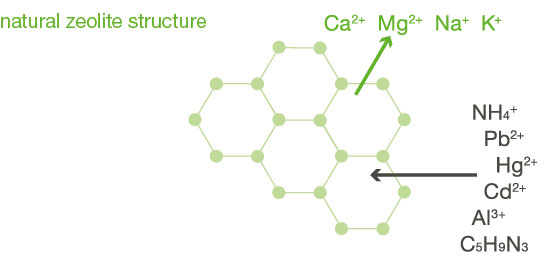
The natural mineral zeolite has been used since ancient times for natural detoxification thanks to its special effect on the human body and is known for its effective pollutant-binding effect (adsorption). We provide pure zeolite as a tested product for internal and external use.
Zeolite Effect - Symptom Relief
Many satisfied users have already experienced the pollutant-binding and draining effect of ZeoliteMED. The accumulation of toxic metals such as mercury, lead, cadmium and aluminium can cause considerable damage to the body and various signs of disease. In particular, allergies, diseases of the gastrointestinal tract such as irritable bowel syndrome and liver dysfunction can occur.
Heavy metal exposure
We often ingest heavy metals without realizing it. Unwashed fruits and vegetables, meat, old lead pipes, contaminated drinking water on holiday or high concentrations of mercury in seafood can put a strain on our bodies and lead to a number of diseases. Zeolite has a detoxifying effect and can protect your body against heavy metals.
Why do humans need help coping with pollutants?
The healthy human body is normally able to independently metabolize toxins in food as well as toxins from the environment to a certain extent. However, certain pre-existing conditions – such as liver damage, allergies or gastrointestinal diseases – can cause the body to become unbalanced and no longer able to cope with the breakdown of harmful substances without problems. In the case of excessive intake of toxins or exposure to particularly toxic substances, such as heavy metals, the body also reaches its limits: diseases occur due to the overload of the organism.
In the presence of pre-existing conditions, it is important to keep the organism as low in pollutants as possible. This can be achieved, for example, through a strictly controlled diet, for example in the form of a special diet. But even with this, it is hardly possible to keep potentially harmful substances, such as lead or cadmium, completely away from the body.
Relieving the liver - keep toxins away
Taking zeolite can have a positive effect on the gastrointestinal tract and also relieve the liver as it is relieved of harmful substances.
Among the substances that natural zeolite can selectively bind are heavy metals such as lead, mercury and cadmium as well as other potentially harmful substances such as aluminium, ammonium and histamine.
Dangers from heavy metals in everyday life
Normally, it is assumed that the risk of suffering from heavy metal poisoning in European countries is relatively low in everyday life. Strict legal requirements give the feeling that it is rather unlikely to come into contact with a dangerous dose of pollutants. However, the reality is different: In fact, almost everyone is exposed to some form of toxic substance on a daily basis. Heavy metals are often ingested directly through food without being noticed.
Where do you find lead?
What about lead, for example? In earlier times, there were quite a few cases of lead poisoning, as the heavy metal was present in everyday life in several places. In the meantime, however, the days of using crockery and cutlery with lead content are over. In addition, unleaded gasoline has established itself as the standard for several decades. And paints mixed with lead are also avoided as much as possible.
Nevertheless, lead can become a health hazard, for example if lead pipes have been installed in a house and they have not yet been replaced. It is also possible that in some households there are still old goblets and spoons circulating (and even being used) in which there are portions of lead. In addition, it can also happen in individual cases to come into contact with lead at work, for example when rust protection paint is handled. And last but not least, beauty care can also make you sick, for example if harmful lead has been used in ointments and creams, e.g. to create a particularly light complexion.
What are the symptoms and dangers of lead poisoning?
Lead poisoning occurs when lead is absorbed into the organism through the gastrointestinal tract, skin or respiratory tract. Depending on the type, duration and intensity of lead exposure, patients have acute or chronic lead poisoning. This particular type of toxication is also known as Saturnism.
Symptoms
Acute lead poisoning manifests itself through symptoms such as headaches, body aches, fatigue and complaints of the gastrointestinal tract.
If the course of the disease is chronic, a so-called lead anemia, i.e. anemia caused by the lead, as well as a lead line on the gums can occur. In addition, other symptoms of chronic lead poisoning include nerve damage (polyneuropathy), disorders of brain functions (encephalopathy), cramping abdominal pain (lead colic), kidney damage and paralysis of the hand (drop hand).
These symptoms, which can be widespread, are due to the fact that lead can damage various organ systems of the body. Depending on the amount ingested, the heavy metal affects the central and peripheral nervous system, the bone marrow (and thus blood formation), the kidneys, the digestive tract, the gonads and the skin, among others. The latter can also lead to chronic lead poisoning in particular being associated with the pale, grey-yellow "lead colour" of the skin.
Dangers
The severity of lead poisoning depends on the level of lead concentration in the blood. People who become acutely severely poisoned can fall into a coma and die of circulatory failure.
After ingestion of lead, it first ends up in the blood, later spreads in the soft tissues and is then partially excreted again. The other part is deposited in bones and teeth in the form of lead phosphate. There it persists with a half-life of up to 20 years. In times when the body breaks down bone substance, elevated blood lead levels can occur even without external influences. Animal studies suggest that lead may also have a carcinogenic effect.
Furthermore, lead is placenta-permeable. That is, it can pass from the mother to the embryo and harm it.
What are the symptoms and dangers of mercury poisoning?
Mercury poisoning is also called mercurialism. The population is usually afraid of coming into contact with mercury vapours through broken fever thermometers or energy-saving lamps. Generally, however, the amount of mercury released in such cases is too small to cause severe poisoning. Nevertheless, old thermometers and energy-saving lamps with mercury must always be treated with vigilance and, in the event of breakage, immediately sealed airtight and the room in which the incident occurs well ventilated as quickly as possible.
A higher risk of mercury poisoning exists for people who work directly with the heavy metal. This can be the case, for example, in laboratories and in the manufacture of thermometers. Mercury has a high vapour pressure - this allows the heavy metal to balance with the atmosphere of a room without sufficient air exchange.
Mercury can be inhaled or absorbed through the skin. It is also possible for the heavy metal to enter the body during ingestion, for example through the consumption of contaminated fish.
Symptoms
The acute form of mercury intoxication begins with symptoms such as headaches, dry mouth, dizziness and nausea and is subsequently characterized by severe vomiting. Untreated, the disease destroys the kidneys and liver.
Sub-acute mercury poisoning leads to symptoms such as increased salivation and inflammation as well as ulcers of the mucous membranes (stomatitis mercurialis). The latter affects in particular the gums, on which a dark seam forms due to the stored mercury sulphide. It is also possible that damage to the intestines and kidneys may occur.
The chronic form of the disease can also be accompanied by stomatitis mercurialis. Especially if the heavy metal has been absorbed in the form of steam, neurological symptoms are the main symptoms. After the mercury vapour enters the body via the lungs, it is transferred to the brain via the blood and the blood-brain barrier. There the oxidation to the mercury ion takes place. The ion then binds to the brain tissue.
During the course of the chronic intoxication, symptoms such as irritability (erethism mercurialis), lack of concentration, sleep problems and intention tremor - i.e. trembling when approaching a target - can occur. Furthermore, chronic mercury poisoning often leads to emaciation (cachexia).
In addition, oral intake of mercury can cause other symptoms such as impaired vision, hearing and walking, paralysis and psychosis.
Dangers
Mercury poisoning can be fatal. For treatment, a drug is administered to remove the heavy metal from the body. Normally, so-called chelates are used for this - however, these have the side effect of also flushing out important minerals and trace elements.
What are the symptoms and dangers of mercury poisoning?
Mercury poisoning is also called mercurialism. There is usually a fear among the population of coming into contact with mercury fumes through broken thermometers or energy-saving lamps. As a rule, however, the amount of mercury released in such incidents is too small to cause severe poisoning. Nevertheless, it is important to always be vigilant when handling old thermometers and energy-saving lamps with mercury and, in the event of breakage, to seal them immediately airtight and to ventilate the room of the incident as quickly as possible.
There is a higher risk of mercury poisoning for people who work directly with the heavy metal. This can be the case, for example, in laboratories and also in the production of thermometers. Mercury has a high vapour pressure, which allows the heavy metal to equilibrium with the atmosphere of a room without sufficient air exchange.
Mercury can be inhaled or absorbed through the skin. Furthermore, it is possible that the heavy metal enters the human body in the course of food intake, for example through the consumption of contaminated fish.
Symptoms
The acute form of mercury intoxication begins with symptoms such as headache, dry mouth, dizziness and nausea and is subsequently characterized by severe vomiting. If left untreated, the disease leads to the destruction of the kidneys and liver.
Subacute mercury poisoning leads to symptoms such as increased salivation and inflammation and ulcers of the mucous membranes (stomatitis mercurialis). The gums are particularly affected by the latter, on which a dark fringe forms due to the stored mercury sulfide. In addition, it is possible for damage to the intestines and kidneys to occur.
The chronic form of the disease can also be associated with stomatitis mercurialis. However, especially if the heavy metal has been ingested in the form of steam, neurological symptoms are more likely to be in the foreground. After the mercury vapor enters the body through the lungs, it is passed through the blood and the blood-brain barrier to the brain. This is where oxidation to the mercury ion takes place. Subsequently, the ion binds to the brain tissue.
During the course of chronic intoxication, symptoms such as irritability (erethism mercurialis), lack of concentration, sleep problems and intention tremor – i.e. trembling occurs when approaching a target – may occur. Furthermore, chronic mercury poisoning often leads to wasting (cachexia).
In addition, oral intake of mercury can cause other symptoms such as impaired vision, hearing, and walking, paralysis, as well as psychosis.
Dangers
Mercury poisoning can be fatal. For treatment, a remedy is administered, with which the heavy metal is removed from the body. As a rule, so-called chelates are used for this - but these have the side effect, among other things, that they can also flush out important minerals and trace elements.
What are the symptoms and dangers of cadmium poisoning?
We encounter cadmium in everyday life from time to time in batteries and accumulators. In addition, humans do not actually come into contact with the heavy metal – at least that's the theory.
In fact, cadmium, like many other toxic substances, is found in a wide variety of foods, such as grains, nuts, cocoa, and legumes. In addition, it is often also found in meat and meat products. It can also be found in chemical products such as fertilisers or pesticides. And other factors such as smoking and house dust can also contribute to the accumulation of too much cadmium in the organism.
Symptoms
Acute cadmium intoxication manifests itself primarily through chemical burns of the digestive tract, which in turn can cause stomach pain, diarrhea and violent vomiting. If the poison has entered the organism by inhalation, it can irritate the respiratory tract. If the exposure was immense, it is also possible that pulmonary edema will develop as a result.
The symptoms of chronic cadmium poisoning include massive pain, which is why the disease is also called "Itai-Itai disease" (= "ouch-ouch" disease) in Japanese. These occur mainly in the back and legs. They are caused by bone softening caused by cadmium, which can lead to spontaneous bone fractures. In addition, damage to the kidneys is likely in the chronic course. Defects in the liver and anemia are also possible. Furthermore, it can cause damage to the central nervous system and the immune system, as well as impaired fertility with potential infertility. Psychological malfunctions, damage to the genetic material, cancer and loss of the sense of smell are also possible symptoms, the latter especially if one has poisoned oneself with the heavy metal by inhalation. Chronic inhaled cadmium poisoning can also lead to the development of pulmonary emphysema.
Dangers
For a long time, it was assumed that cadmium could not poison oneself at all. The first poisoning with the heavy metal was described only in 1858. In fact, the human body has a special protein that is able to bind excess cadmium. So far, there is no specific treatment for the disease.
Reducing the potential danger with zeolite
Every day, humans are confronted with a variety of harmful substances, be it toxic heavy metals or other potentially toxic substances.
A healthy body can cope with a small amount of harmful substances on its own and detoxify them through the body's own digestion. However, it becomes problematic when the organism is flooded with too many toxins or has certain pre-existing conditions. Then the body's own detoxification functions are overwhelmed and faulty processes occur in which toxic substances accumulate in the body and can cause serious damage.
Zeolite can help the body cleanse the gastrointestinal tract of harmful substances and thus help to relieve important detoxification organs such as the liver and kidneys.
Zeolite Powder, Ultra Fine Powder or Capsules?
Zeolite is available in different grain sizes as powders, ultra-fine powders and capsules.
Powder
Powder can be easily stirred or whisked in water and drunk. It is already in contact with the oral mucosa, so that the pollutant-binding properties can work throughout the digestive system.
Ultra Fine Powder
The ultra-fine powders are even smaller or finer micronized than powders. As a result, they have an even larger internal surface area and can thus bind more pollutants, which is why it is dosed less than the powder. The ultra-fine powders are completely without "crumbs" and so fine that you feel as if they are dissolving.
Encapsulate
The purely plant-based (vegan) capsules are the ideal companion for on the go because of their simple and practical use. Capsules are taken with water and are an alternative for people who are less fond of the taste of the natural mineral.
Effect of Zeolite Natural Cosmetics (external use)
Zeolite-clinoptilolite is now an integral part of natural cosmetics. The unique effect of the volcanic mineral zeolite is already used by us in almost all natural cosmetic products. Zeolite acts as a filter on your skin, binding harmful toxins that would otherwise irritate and inflame your skin unhindered.
Zeolite Fluoride Free Toothpaste
Zeolite also has a positive effect in toothpaste. With the help of zeolite, impurities and plaque are gently and effectively removed. Fluoride, which can be very harmful and even dangerous for humans in high doses, is not necessary.
Zeolite Skin Cream
Detoxification on the skin supports the skin's regeneration process. Zeolite works by binding harmful substances and thus relieving the skin. The daily use of the rich zeolite skin cream for all skin types is an integral part of the daily natural cosmetics routine.
Zeolite interactions with other agents
After taking medication, an interval of at least 2 hours should be maintained. Do not use at the same time as alcoholic, caffeinated and acidic beverages such as grapefruit, orange, lemon and pineapple juice.
Side effects of Zeolite
In rare cases constipation can occur as a side effect of Zeolite due to low fluid intake. This effect is dependent on dosage and can be avoided through adequate fluid intake and dosage reduction. In the occurence of constipation, increase fluid intake, reduce dosage and, if necessary, consult your doctor.
Warnings and precautions
In order to support the detoxification and regulation processes, it is necessary to ensure abundant hydration during the intake of ZeoliteMED, both in terms of intake (0.25 liters of water) and in relation to the daily ration (2-3 liters/day).
Patients with constipation or impaired renal function should only use it after the consultation with a doctor. This also applies to drug therapy, chemotherapy or radiotherapy.
Pregnant, breastfeeding, children and adolescents (under 18 years of age) must contact their doctor before use.
Not intended for continuous use. Discontinue use if you notice an unusual effect.
Application and dosage of zeolite
Dosage and duration of zeolite detoxification
Zeolite can be used individually over days, weeks or months, depending on your needs. Please pay close attention to the instructions for use on the respective product (label).
Dosage of zeolite when used internally
Powders and ultrafine powders can simply be stirred into a glass of water (250 ml) and drunk with a meal. The dosage varies depending on the mineral chosen and the degree of fineness. Please refer to the instructions in the respective instructions for use, which can be found directly on the label.
Dosage of zeolite when used externally
External application examples:
- Zeolite is also often used in powder form as a foot powder or body powder.
- Zeolite as a bath additive for foot baths (1 tsp) and full baths (1 tbsp)
- You can also mix zeolite as a pack with water in a pulp-like manner, apply it to affected areas, cover it with compresses and let it work overnight, for example
- Zeolite for use in skin care: take a pinch of ultra-fine powder in the palm of your hand, mix with skin care cream and apply as usual
- Zeolite for masks: Mix 1 tsp powder/powder with a little water, apply thinly, rinse after 10 - 15 min.
First use of zeolite and/or sensitive?
Experience has shown that in case of high sensitivity, first-time use or longer breaks, the application with lower dosages should be slowly creeped in with 1x daily. and if necessary also slowly to 2x daily. up to max. 3x daily be increased.
What is zeolite? A chemical description.
Natural zeolite is usually composed of the two silicon minerals heulandite or clinoptilolite. In addition to these two main components, crystalline aluminosilicate contains other minerals as well as other trace elements. The so-called primary components of naturally occurring zeolite are AIO4 and SiO4 tetrahedrons. These are connected to neighboring oxygens ("O") and therefore have a crystalline framework structure in three-dimensional form. At the same time, the structure of zeolite has the property of possessing a highly developed system of cavities and microchannels. This is the so-called "porosity of zeolite/clinoptilolite".
The Structure
Zeolite consists of a regularly shaped lattice framework and contains both alkali and noble alkali cations. In the cavities of natural zeolite mentioned above, cations and water molecules are stored. Due to their high mobility, both the cations and H2O molecules have the ability for a pronounced ion exchange. The size of the channels in the crystal structure of zeolite is sufficient for foreign molecules and ions to enter. With this property, zeolite is considered an effective ion exchanger, heat storage and molecular sieve in the field of science. Basically, zeolite has a relatively impressive surface, which means that it brings great added value in various areas of application. The chemical formula for the crystalline aluminosilicate is as follows:
M2/n Al2O3 x SiO2 yH2O
The structure in detail
From a chemical point of view, this crystalline aluminosilicate is a salt. While the anions have a lattice-like structure, the cations form individual particles. Within the tetrahedron, positive charge of the contained aluminum ion is usually completely compensated by negative charge of three of the four oxygen atoms present. The fourth atom must be balanced with the help of an alkali or alkaline earth metal cation. Na+/K+ or Ca2+ or Mg2+ are usually used for this purpose. The cations settle close to the negatively charged tetrahedrons within the cavities and channels of the silicate. The channel windows of this silicate have a diameter of 0.3 and 0.6 nanometers.
Chemistry: Zeolite Clinoptilolite
Zeolite and clinoptilolite compounds are among the materials most commonly used in modern society. Even if you are not aware of it, you will encounter the numerous applications of zeolite and clinoptilolite in everyday life again and again. For us, this is one of the best reasons to take a closer look and take a closer look at zeolite and clinoptilolite or zeolite and clinoptilolite compounds:
Family ties zeolite and clinoptilolite
Both are assigned to the mineral class "silicates and germanates" - with clinoptilolite compounds forming a subgroup of zeolite. In accordance with this classification, you will first find explanations of the latter substances at this point, from which the transition to clinoptilolite results more or less by itself.
Zeolite: Nomen est omen
Zeolite got its name from the Swedish-born mineralogist Baron Axel Fredrick von Cronstedt, who in the mid-18th century intensively studied the composition and properties of the substance. One insight gained from his observations was reflected in the name the scientist chose for his research object: The term "zeolite" coined by him is based on the ancient Greek words "zeein" for "boiling" and "lithos" for "stone" - with which Baron von Cronstedt described the lively effervescence that can be observed when the mineral is heated.
Zeolite: The Secret of the "Boiling Stone"
It comes from the water contained in the crystal structure of zeolite, which is released again under the influence of heat. The basic structure, which consists of aluminum and silicon atoms, is completely preserved during this process, as its individual components are held together exclusively by oxygen atoms and the liquid is only located in the intervening cavities. They give zeolite a strikingly large "inner surface area" that can be up to more than 1,000 m² per gram of material.
Zeolite: Based on nature's example
The ability of the special structure to absorb and release other substances makes zeolite a natural screening and filtering system. In line with this function, it acts as a catalyst or separator in numerous biochemical processes. In order to be able to use this property on a larger or industrial scale, zeolite has been produced synthetically in increasing quantities since 1950. For this purpose, alkaline solutions based on aluminum and silicon compounds are used, which crystallize at temperatures from 60°C. The zeolite obtained in this way can be modified by the exchange of individual ions or by chemical post-treatment in such a way that its catalytic effect, absorption and storage capacity as well as chemical or thermal stability are greatly increased.
Zeolite: A wide range of applications
Examples of the use of natural or synthetic zeolite and its modifications can be found in many areas. It is used, among other things, in the separation of chemical substances, in heat storage heating systems and as a nitrogen absorber in large-scale industrial plants. In everyday life, you will encounter zeolite as an ingredient in dishwashing and laundry detergents, where it supports the drying process on the one hand and serves as a water softener on the other. In addition, it is offered in zoological retailers for cleaning or keeping aquarium and pond water clean.
Zeolite: A Late(er) Discovered Sprout
Similar applications can be found for clinoptilolite. As a subgroup of zeolite, it has approximately the same structure and correspondingly identical properties. In contrast to its superior zeolite, however, clinoptilolite is already naturally present in compounds containing calcium, potassium or sodium ions. Such a compound mineral was first detected in 1923 at Hoodoo Mountain in North America. Its discoverer - the American mineralogist Waldemar Theodore Schaller - initially considered it to be an independent representative of its species. It was only in subsequent analyses that it turned out that the clinoptilolite found in the state of Wyoming is closely related to the already known zeolite.
Zeolite: condition and color variable
The mineral, which is normally colourless and completely transparent, is present in fine grains as well as in the form of tabular and massive crystals. Depending on how evenly these are structured or whether they contain admixtures, the tint of clinoptilolite can tend to white or have a yellowish to reddish-white tinge.
Zeolite: Just as versatile and a little bit more
According to the membership of the zeolite group, clinoptilolite compounds with calcium, potassium or sodium ions have the same biochemical and physical properties as the classifying mineral. They are also used for filter and cleaning purposes in industrial plants, in the production of construction and fuel or as release agents. Due to its natural structure, however, clinoptilolite enriched with calcium, potassium or sodium finds even more applications. Thus, the members of the zeolite group are needed wherever synthetic additives are undesirable or even harmful. Examples of this are its use as a fertiliser in gardening and landscaping, as an odour binder and feed additive in livestock breeding or in the context of environmental care.
Zeolite: Reliable help in an emergency
Clinoptilolite made its most popular appearance in this regard after the Chernobyl nuclear disaster. After the meltdown in the nuclear reactor of the Russian city severely polluted the air as well as the waters and soil in the surrounding area, experts used the natural filter clinoptilolite. It was not only used to clean rivers and sewage pipes, but was also added to livestock feed. In the digestive tract of the cows, sheep, goats and horses supplied with it, the clinoptilolite compounds acted as ion exchangers and helped to bind radioactive substances in the animals' bodies.
Zeolite: Tried and tested reapplied
The success of these measures has been so great that they have established themselves as an effective means of combating such incidents. For example, clinoptilolite was also used in the Japanese reactor at Fukushima after the nuclear disaster. Here, with the help of the natural filtering and adsorption properties of zeolite and clinoptilolite, inlets to the sea were decontaminated in order to avoid further environmental pollution by radioactive contamination.
Zeolite: A substance that warms and cools in equal measure
The heat generated during these and similar processes is a popular "by-product" of the application. For example, zeolite and clinoptilolite compounds can be used simultaneously to operate heating systems. However, they find a much more attractive use in the opposite case - the beer keg with automatic cooling, which is popular during the warm summer months. Here, too, the adsorption capacity of zeolite or clinoptilolite and the associated driving force are used. When buying the next barrel, just pay a little more attention to the labeling - after all, you now know what the secret of the dewy and well-chilled barley juice lies in: in the principle of action of the minerals zeolite and clinoptilolite.












